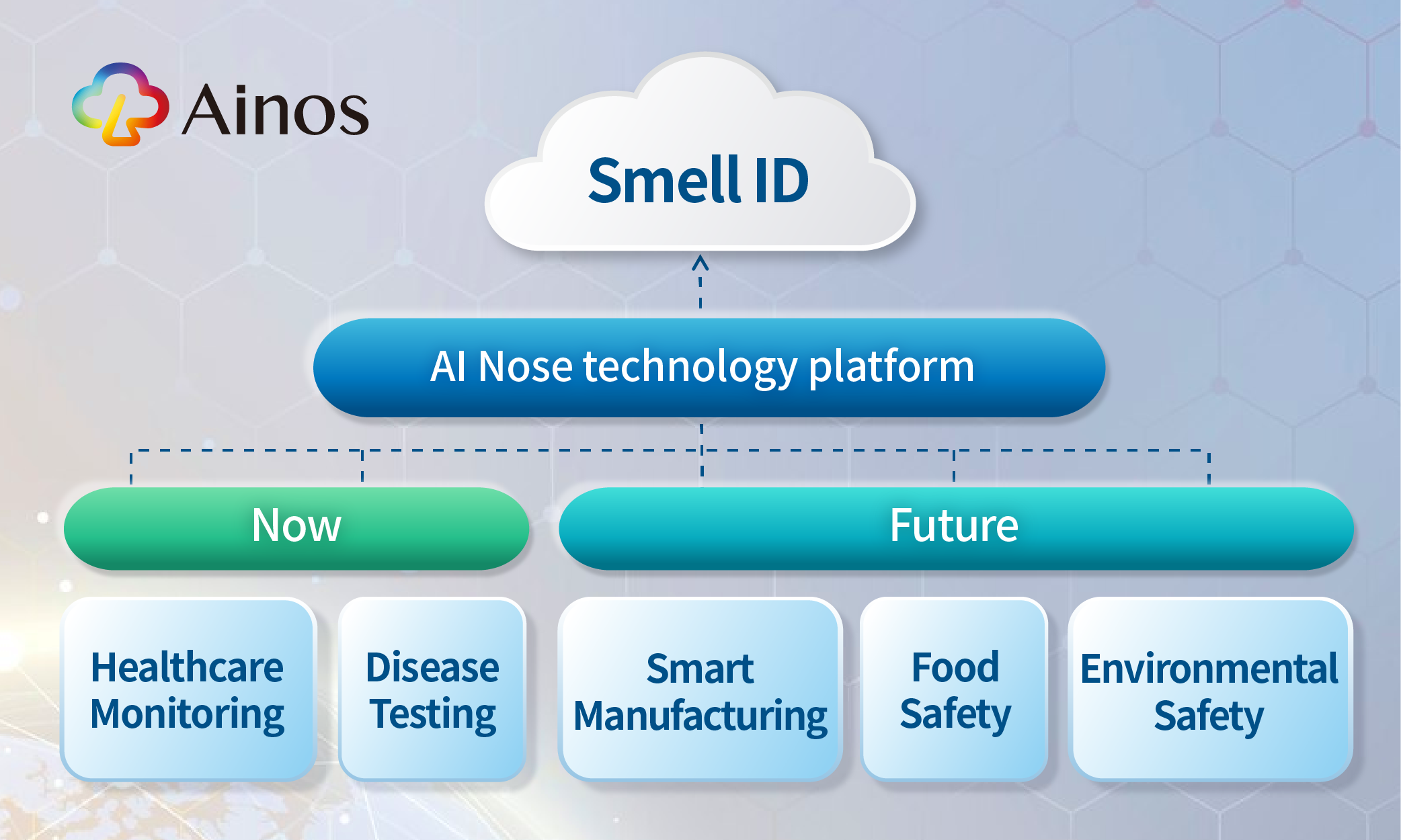
Embarking on a journey to ultimately becoming AI's nose capable of digitizing smell
AI Nose journey begins in healthcare and is now expanding into smart factories
SAN DIEGO, CA / ACCESSWIRE / August 26, 2024 / Ainos, Inc. (NASDAQ:AIMD)(NASDAQ:AIMDW) ("Ainos", or the "Company"), a diversified healthcare company focused on novel AI-powered point-of-care testing ("POCT") and VELDONA low-dose interferon therapeutics, is pleased to provide an update letter to shareholders from its Chairman of the Board, President, and Chief Executive Officer, Mr. Chun-Hsien (Eddy) Tsai.
Dear Shareholders,
Since rebranding to Ainos, Inc in 2021, our team has worked tirelessly to unlock the full potential of our AI Nose technology. We are on a mission to leverage digital nose sensors and our proprietary volatile organic compounds ("VOC") sensing AI algorithm, to digitize smell and ultimately become AI's nose. Over the past 12 months, I'm proud to say that we have made many significant achievements, and I'd like to recap these milestones as well as our strategies.
Our Endgame: To Become AI's Nose, Starting with Healthcare
We embark our smell digitalization journey from the healthcare market. Looking ahead into the near future, we believe the underlying mega-trend is that as population ages, increasing demand for healthcare will collide with a labor shortage, potentially compromising the quality of health tests. We believe telehealth-friendly point-of-care tests ("POCTs") will be crucial in reducing the burden on healthcare workers.
We anticipate that VOC could be powerful biomarkers for detecting diseases and enabling easy, efficient health monitoring. Powered by AI Nose, we are making POCTs more consumer-friendly, telehealth-compatible, AI-driven, and poised to benefit more people. AI Nose will become increasingly accurate as we train the platform with more data.
Empowering Women's Health and Improving Tests for Sexually Transmitted Infections ("STIs"): Next-Gen Ainos Flora Clinical Study Kickoff in Q4 2024
Our lead product candidate, Ainos Flora, targets women's health and certain common STIs. I believe this market is underserved: about more than 1 million new STIs occur globally every day, and vaginal infections such as bacterial vaginosis (BV) increase risks of STIs. Young people (15-24 year olds) are estimated to account for nearly half of new STI infections in America. POCT is estimated to account for only 31% of the total STI tests. We aim to address these key hurdles to regular screening: convenience, privacy and affordability.
Ainos is developing the next-gen Ainos Flora, optimized for at-home testing. We are fast-tracking development by utilizing NVIDIA CUDA's accelerated computing, targeting Q3 2024 for design completion and Q4 2024 for clinical trial kickoff. We now explore to position the first-gen Ainos Flora for the hospital and clinic market. Our product strategy places Ainos Flora across key use scenarios.
Elderly Care: On Track to Pilot Production in Q4 2024
In August 2023, we kicked off a VOC sensing co-development with Nisshinbo Micro Devices Inc. ("NISD") and Taiwan Inabata Sangyo Co. ("Inabata"). Our shared goal is to broaden addressable market of AI Nose VOC sensing to include telehealth, automotive, industrial, and environmental safety applications.
Our first project focuses on elderly care. The crucial megatrend is that as the global population ages, the increasing demand for elderly care will clash with a labor shortage, leading to compromised care quality. To address this, Ainos and our partners aim to automate certain health monitoring tasks with an AI-driven, telehealth-friendly solution powered by AI Nose VOC sensing.
We plan to first commercialize this product in Japan by collaborating with our co-developers. The population in Japan is aging more rapidly than any other country, with about 36 million Japanese citizens aged 65 and over at almost a third of its population. We are on track to pilot production in 2H 2024.
First Move Beyond Healthcare: AI Nose in Smart Factories; Pilot Production Ready in Q4 2024
Our second co-development program marks our first step in extending AI Nose beyond healthcare. We recognize that smart manufacturing or smart factory requires 1) more AI-driven sensors to capture and analyze data; 2) increased robotic automation to tackle a shrinking workforce. While AI-driven visual data is already well established, AI-driven VOC sensing is still in its early stages. This is evidenced by the lack of digital noses in today's existing humanoid robots. AI Nose can equip robots with a digital sense of smell, enhancing their ability to collaborate with human workers. This improves robot performance and ensures a safer work environment.
We also see vast potential for AI Nose in the semiconductor manufacturing factories, as it has the potential for detecting leaks of photoresist materials, hazardous chemicals and overheating cables.
For now, we are thrilled to announce that AI Nose is 79% accurate in identifying 761 samples across 22 VOCs in Japanese semiconductor manufacturing factories. This validates AI Nose's capabilities in the smart factory market. We target to be pilot production ready in Q4 2024.

New Licensing Strengths AI Nose and POCT IPs
As we advance the previously mentioned programs, we are also strengthening our IP portfolio. We have recently secured exclusive licenses for 10 multi-region invention patents and patent applications surrounding gas sensors and medical device technologies from our product co-developer and sole manufacturer of our POCTs. We completed the transaction with an all-stock structure valued at approximately $5.4 million, or $0.99 per share, which is at a premium to the market price. This structure algins our partner' interests with those of our shareholders and enables us to preserve cash for executing our programs.
Looking Ahead: Confidence in Our Future
As we reflect on our achievements and strategic moves, we remain profoundly optimistic about what the future holds. The milestones we've reached and the strategic programs we are advancing reinforce our confidence in the transformative potential of AI Nose technology.
We are poised to leverage our VOC technological advancements or strategic partnerships to drive significant impact across multiple aspects, from enhancing POCTs for better women's health to revolutionizing elderly care as well as expansion into smart manufacturing. We believe that the progress we have made positions us well for continued growth and success.
As we move forward, we remain focused on executing our plans with precision and agility, continuously pushing the boundaries of innovation. We are excited about the opportunities ahead and we have confidence in our strategic initiatives for empowering our mission of digitizing smell across diverse scenarios.
Thank you for your continued support in our journey.
Key AI Nose Milestones
On August 19, 2024, we announced that AI Nose is has a 79% accuracy rate in identifying 761 samples across 22 VOCs in Japanese semiconductor manufacturing factories. This demonstrates AI Nose's potential to revolutionize the smart manufacturing factory automation and manufacturing safety. We are also planning to integrate AI Nose into robotics systems in smart factories.
On August 9, 2024, we announced that we obtained exclusive licenses for 10 invention patents and patent applications to bolster our AI Nose and POCT technologies. The licensed patents and applications surround gas sensors and medical devices technologies and cover the U.S., Germany, China, Japan and Taiwan. We completed the licensing with an all-stock transaction valued at approximately $5.4 million, or $0.99 per share.
On June 14, 2024, we announced that we will advance the next-gen Ainos Flora with the implementation of NVIDIA CUDA, leveraging critical insights from first-gen Ainos Flora's clinical trials over 75 subjects at four Taiwanese medical centers.
On December 26, 2023, Ainos, NISD and Inabata initiated phase 2 of VOC co-development powered by AI Nose, propelling AI Nose towards broader industry adoption and advancing the mission of digitalizing smell.
On August 9, 2023, we announced that Ainos partnered with NISD and Inabata to co-develop VOC sensing platform powered by AI Nose, kicking off our goal for broader AI Nose industry adoption, and progressing further in our mission for digitizing smell.
About Ainos, Inc.
Headquartered in San Diego, California, Ainos is a diversified healthcare company focused on novel AI-powered point-of-care testing (POCT) and low-dose interferon therapeutics (VELDONA). The Company's clinical-stage product pipeline includes VELDONA human and animal oral therapeutics, human orphan drugs, and telehealth-friendly POCT solutions powered by its AI Nose technology platform.
The name "Ainos" is a combination of "AI" and "Nose" to reflect the Company's commitment to empowering individuals to manage their health more effectively with next-generation AI-driven POCT solutions. To learn more, visit https://www.ainos.com.
Follow Ainos on X, formerly known as Twitter, (@AinosInc) and LinkedIn to stay up-to-date.
Safe Harbor Statement
Certain statements in this press release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact are forward-looking statements. Forward-looking statements can be identified by the use of words such as "anticipate," "believe," "estimate," "approximate," "expect," "intend," "plan," "predict," "project," "target," "future," "likely," "strategy," "foresee," "may," "guidance," "potential," "outlook," "forecast," "should," "will" or other similar words or phrases. Similarly, statements that describe the Company's objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements are based only on the Company's current beliefs, expectations, and assumptions. Forward-looking statements are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict and many of which are outside of the Company's control. The Company's actual results may differ materially from those indicated in the forward-looking statements.
Important factors that could cause the Company's actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release include, among others, the cost of production and sales potential of the products announced in this press release; the Company's dependence on projected revenues from the sale of current or future products ; the Company's limited cash and history of losses; the Company's ability to achieve profitability; the Company's ability to raise additional capital to continue the Company's product development; the ability to accurately predict the future operating results of the Company; the ability to advance Ainos' current or future product candidates through clinical trials, obtain marketing approval and ultimately commercialize any product candidates the Company develops; the ability to obtain and maintain regulatory approval of Ainos' product candidates; delays in completing the development and commercialization of the Company's current and future product candidates, which could result in increased costs to the Company, delay or limit the ability to generate revenue and adversely affect the business, financial condition, results of operations and prospects of the Company; intense competition and rapidly advancing technology in the Company's industry that may outpace its technology; customer demand for the products and services the Company develops; the accuracy of third-party market research data, the impact of competitive or alternative products, technologies and pricing; disruption in research and development facilities; lawsuits and other claims by third parties or investigations by various regulatory agencies governing the Company's operations; potential cybersecurity attacks; increased requirements and costs related to cybersecurity; the Company's ability to realize the benefits of third party licensing agreements; the Company's ability to obtain and maintain intellectual property protection for Ainos product candidates; compliance with applicable laws, regulations and tariffs; continued listing on and compliance with the applicable regulations of the Nasdaq Capital Market; and the Company's success in managing growth. A more complete description of these risk factors and others is included in the "Risk Factors" section of Ainos' Annual Report on Form 10-K for the year ended December 31, 2023, and other public filings with the U.S. Securities and Exchange Commission ("SEC"), many of which risks are beyond the Company's control. In addition to the risks described above and in the Company's filings with the SEC, other unknown or unpredictable factors also could cause actual results to differ materially from the projections, forecasts, estimates and expectations discussed in this press release.
The forward-looking statements made in this press release are expressly qualified in their entirety by the foregoing cautionary statements. Any forward-looking statements contained in this press release represent Ainos' views only as of today and should not be relied upon as representing its views as of any subsequent date. Ainos undertakes no obligation to, and expressly disclaims any such obligation to, publicly update or revise any forward-looking statement to reflect changed assumptions, the occurrence of anticipated or unanticipated events or changes to the future results over time or otherwise, except as required by law.
Investor Relations Contact
Feifei Shen
Email: [email protected]
SOURCE: Ainos, Inc.
View the original press release on accesswire.com