Edgewise Therapeutics, Inc., (Nasdaq: EWTX), a leading muscle disease biopharmaceutical company, today announced top-line data of EDG-7500 from the Phase 1 trial in healthy subjects and the single-dose arm of the Phase 2 CIRRUS-HCMtrial in patients with obstructive HCM. EDG-7500 is a novel oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with HCM.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240919115598/en/
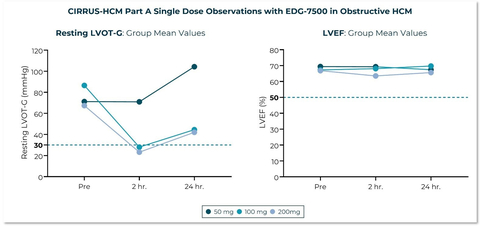
CIRRUS-HCM Part A Single Dose Observations with EDG-7500 in Obstructive HCM (Graphic: Business Wire)
In the placebo-controlled Phase 1 single ascending dose (SAD) trial (n=48), healthy subjects received single doses of EDG-7500, ranging from 5 to 300 mg. In the multiple ascending dose (MAD) portion of the trial (n=24), healthy subjects received 25 to 100 mg once daily for 14 days. EDG-7500 was well tolerated in both the SAD and MAD; there were no clinically meaningful changes or trends in vital signs, clinical chemistry, hematology, or electrocardiograms. There were no meaningful changes in LVEF for all SAD and MAD subjects across a broad range of EDG-7500 exposures. In the MAD, a half-life of approximately 30 hours was observed, and steady state was achieved in approximately 4 days after the start of once-daily dosing. Generally, dose proportional increases in exposure were observed in both SAD and MAD.
In CIRRUS-HCM Part A, patients with obstructive HCM received a single dose of 50, 100 or 200 mg of EDG-7500. A 67% mean reduction in resting LVOT pressure gradient (LVOT-G) and a 55% mean reduction in provokable (Valsalva) LVOT-G were observed in patients receiving the 100 and 200 mg single doses. LVOT gradients less than 30 mmHg at rest and less than 50 mmHg with Valsalva were observed in 60% of patients receiving 100 or 200 mg of EDG-7500. Importantly, gradient reduction was achieved without a meaningful change in LVEF. Treatment with a single dose of EDG-7500 also led to a 64% mean reduction in NT-proBNP, a key biomarker of heart failure, in the 200 mg cohort. This reduction highlights the potential of our mechanism in the treatment of diseases of diastolic dysfunction, including non-obstructive HCM.
Across the Phase 1 and CIRRUS-HCM trials, no subjects had a LVEF reduction to below 50% across a broad range of EDG-7500 exposures.
“Based on the strength of clinical and preclinical data to-date, we have initiated the 28-day part of CIRRUS-HCM in patients with obstructive and non-obstructive HCM,” said Marc Semigran, M.D., Chief Development Officer, Edgewise Therapeutics. “Importantly, we plan to continue the evaluation of tolerability, pharmacokinetics and effects on LVOT-G, LVEF, biomarkers and measures of feel and function in these patients.”
Anjali T. Owens, M.D., Medical Director, Center for Inherited Cardiac Disease, Associate Professor of Medicine, University of Pennsylvania and CIRRUS-HCM Investigator highlighted, “There continues to be an unmet need for patients with obstructive and non-obstructive HCM, and we are excited to be part of the ongoing CIRRUS-HCM trial evaluating a novel treatment.”
Kevin Koch, Ph.D., President and Chief Executive Officer, Edgewise Therapeutics added, “We believe our innovative approach, where we have observed gradient relief without reductions in LVEF, has the potential to be a valuable advancement in the treatment of obstructive HCM. We expect to report initial 28-day data in the first quarter of 2025.”
EDG-7500 Topline Data Webcast Event
Members of the Edgewise management team will hold a live webcast on Thursday, September 19, 2024, at 8:30 am ET to discuss the top-line data, and will be joined by leading cardiology expert, Anjali T. Owens, M.D., Medical Director, Center for Inherited Cardiac Disease, Associate Professor of Medicine, University of Pennsylvania, who will share her perspective of EDG-7500 and HCM. An accompanying slide presentation will also be available. To register for the live webcast and replay, please visit the Edgewise events page.
About EDG-7500
EDG-7500 is a novel oral, selective, cardiac sarcomere modulator, specifically designed to slow early contraction velocity and address impaired cardiac relaxation associated with hypertrophic cardiomyopathy (HCM) and other diseases of diastolic dysfunction. Preclinical data in models of both obstructive and non-obstructive HCM suggest the ability to drive a beneficial response at a low risk of decreasing left ventricular ejection fraction below normal. The Company is enrolling CIRRUS-HCM, a three-part, multi-center, open-label trial, in approximately 55 patients with HCM at up to 20 clinical sites in the U.S. The primary objective of Part A of the trial was to evaluate the safety and tolerability of a single oral dose of EDG-7500. Other key outcome measures included pharmacokinetics (PK), LVEF, and resting and provocable LVOT-G. Parts B and C will evaluate multiple doses of EDG-7500 over 28-days in patients with obstructive or non-obstructive HCM.
To learn more about CIRRUS-HCM, visit clinicaltrials.gov, NCT06347159 (Phase 2).
About Hypertrophic Cardiomyopathy
Hypertrophic Cardiomyopathy (HCM) is the most common form of genetic heart disease, affecting approximately one in 500 people, and is associated with reduced quality of life and an elevated risk of heart failure, abnormal heart rhythms, and sudden cardiac death (SCD). Individuals with HCM can become extremely limited in their functional capacity and ability to perform the activities of daily living. Commonly experienced symptoms include breathlessness, irregular heartbeats, chest pain, tiredness, dizziness, or even fainting. These symptoms are caused by excessive contraction and thickening (hypertrophy) of the left ventricular wall of the heart. Over time, the thickened muscle becomes stiff, making it difficult for the heart to relax and fill with blood (diastolic dysfunction). There are two major forms of HCM obstructive and non-obstructive. The obstructive HCM pathology is observed in two thirds, while non-obstructive HCM is present in one third of all individuals with HCM. Despite advancements in treatment options for some HCM patients, there remains a significant unmet need for additional therapeutic approaches for patients.
About Edgewise Therapeutics
Edgewise Therapeutics is a leading muscle disease biopharmaceutical company developing novel therapeutics for muscular dystrophies and serious cardiac conditions. The Company’s deep expertise in muscle physiology is driving a new generation of novel therapeutics. Sevasemten is an orally administered skeletal myosin inhibitor in late-stage clinical trials in Becker and Duchenne muscular dystrophies. EDG-7500 is a novel cardiac sarcomere modulator for the treatment of hypertrophic cardiomyopathy and other diseases of diastolic dysfunction, currently in Phase 2 clinical development. The entire team at Edgewise is dedicated to our mission: changing the lives of patients and families affected by serious muscle diseases. To learn more, go to: www.edgewisetx.com or follow us on LinkedIn, X, Facebook and Instagram.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, statements regarding the potential of, and expectations regarding EDG-7500; statements regarding the timing of reporting data (including the data from the CIRRUS-HCM 28-day trial); statements regarding Edgewise’s expectations relating to its clinical trials (including the Phase 2 trial of EDG-7500 in individuals with obstructive HCM, the CIRRUS-HCM 28-day trial, and open-label extension trial of EDG-7500); statements regarding the commencement of trials (including the open-label extension trial of EDG-7500); and statements by Edgewise’s president and chief executive officer and chief development officer. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements. The forward-looking statements contained herein are based upon Edgewise’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results could differ materially from those projected in any forward-looking statements due to numerous risks and uncertainties, including but not limited to: risks associated with Edgewise’s limited operating history, its products being early in development and not having products approved for commercial sale; risks associated with Edgewise not having generated any revenue to date; Edgewise’s ability to achieve objectives relating to the discovery, development and commercialization of its product candidates, if approved; Edgewise’s need for substantial additional capital to finance its operations; Edgewise’s substantial dependence on the success of its sevasemten; Edgewise’s ability to develop and commercialize sevasemten and EDG-7500 and discover, develop and commercialize product candidates in future programs; risks related to Edgewise’s clinical trials of its product candidates not demonstrating safety and efficacy; risks related to Edgewise’s product candidates causing serious adverse events, toxicities or other undesirable side effects; the outcome of preclinical testing and early clinical trials not being predictive of the success of later clinical trials and the risks related to the results of Edgewise’s clinical trials not satisfying the requirements of regulatory authorities; delays or difficulties in the enrollment and/or maintenance of patients in clinical trials; risks related to failure to capitalize on other indications or product candidates; risks related to competition; risks relating to interim, topline and preliminary data from Edgewise’s clinical trials changing as more patient data becomes available; risks related to the regulatory approval processes being lengthy, time consuming and inherently unpredictable; risks related to regulatory authorities not accepting data from trials conducted in locations outside of their jurisdiction; risks relating to Edgewise’s ability to attract and retain highly skilled executive officers and employees; Edgewise’s ability to obtain and maintain intellectual property protection for its product candidates; Edgewise’s reliance on third parties; general economic and market conditions; and other risks. Information regarding the foregoing and additional risks may be found in the section entitled “Risk Factors” in documents that Edgewise files from time to time with the U.S. Securities and Exchange Commission. These forward-looking statements are made as of the date of this press release, and Edgewise assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
This press release contains hyperlinks to information that is not deemed to be incorporated by reference into this press release.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240919115598/en/